Embark on an exploration of history of an atom worksheet answers, delving into the fascinating world of atomic physics. This comprehensive guide unveils the fundamental principles that govern the structure, behavior, and applications of atoms, providing a solid foundation for understanding the building blocks of our universe.
From the groundbreaking discoveries of Dalton and Thomson to the revolutionary models of Rutherford and Bohr, we trace the evolution of atomic theory, highlighting the key experiments and observations that shaped our understanding of the atom.
History of the Atomic Model
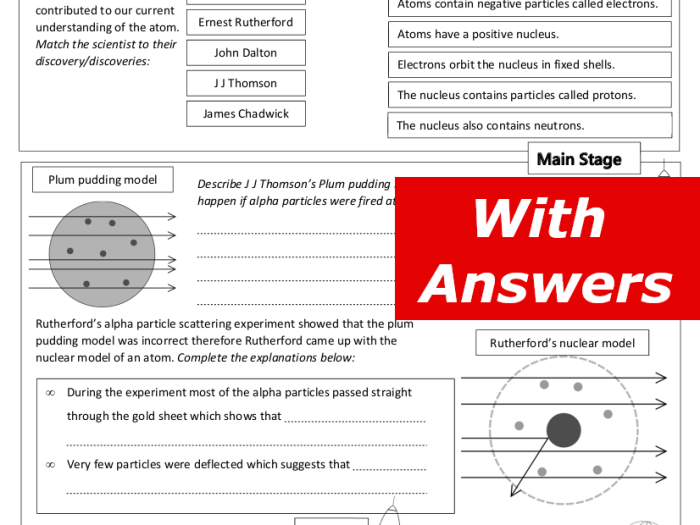
The development of the atomic model has been a continuous process of scientific discovery and experimentation. The key discoveries that led to our current understanding of the atom include:
- Dalton’s atomic theory (1803):Proposed that matter is composed of indivisible atoms and that all atoms of an element are identical.
- Thomson’s discovery of the electron (1897):Showed that atoms are not indivisible and that they contain negatively charged particles called electrons.
- Rutherford’s gold foil experiment (1911):Demonstrated that atoms have a small, dense nucleus that contains most of the atom’s mass.
- Bohr’s atomic model (1913):Proposed that electrons orbit the nucleus in discrete energy levels.
Structure of an Atom
An atom consists of a nucleus and electrons. The nucleus is located at the center of the atom and contains protons and neutrons. Protons have a positive charge, while neutrons have no charge. The number of protons in an atom determines its atomic number, which is unique for each element.
Electrons are located in shells around the nucleus. Each shell has a specific energy level. Electrons in higher energy levels are more loosely bound to the nucleus than electrons in lower energy levels.
Chemical Properties of Atoms: History Of An Atom Worksheet Answers
The chemical properties of an atom are determined by the number and arrangement of its electrons. Valence electrons are the electrons in the outermost shell of an atom. These electrons are involved in chemical bonding.
Atoms with similar valence electron configurations have similar chemical properties. This is the basis of the periodic table, which organizes elements by their atomic number and chemical properties.
Nuclear Reactions
Nuclear reactions are reactions that involve the nucleus of an atom. These reactions can release or absorb energy. The two main types of nuclear reactions are radioactive decay and nuclear fission.
Radioactive decay is a process in which an unstable atom emits particles and energy to become more stable. Nuclear fission is a process in which a heavy atom splits into two or more smaller atoms, releasing energy.
Applications of Atomic Physics

Atomic physics has a wide range of applications in various fields, including medicine, energy, and materials science.
- Medicine:Atomic physics is used in medical imaging techniques such as X-rays and MRI scans. It is also used in the development of new cancer treatments.
- Energy:Atomic physics is used in the development of nuclear power plants and other energy technologies.
- Materials science:Atomic physics is used in the development of new materials with improved properties, such as strength and durability.
FAQ Overview
What is the basic structure of an atom?
An atom consists of a nucleus, which contains protons and neutrons, surrounded by electrons orbiting in energy levels.
How do electrons determine the chemical properties of an atom?
The arrangement of electrons in an atom’s outermost energy level, known as valence electrons, determines its chemical reactivity and bonding behavior.
What is nuclear fission?
Nuclear fission is a process in which a heavy atomic nucleus splits into two or more lighter nuclei, releasing a significant amount of energy.